BSICoS focuses its activity on the Processing, Interpretation and Computer Simulation of Biomedical Signals.
BSICoS: Biomedical Signal Interpretation and Computational Simulation
The main objective of the group is the development of methods for biomedical signal processing, driven by the physiology, for personalized interpretation (diagnosis, prognosis and therapy) of the conditions of the cardiovascular, respiratory and autonomic nervous systems and their interactions.
The goal is to improve the impact of ICTs in health and further understanding the functioning of biological systems that can be observed through noninvasive signals.
Key to this is working with clinical teams and research groups that combine the experiences of the two areas, direct research to solve relevant clinical problems and facilitate the transfer of results to clinical practice.
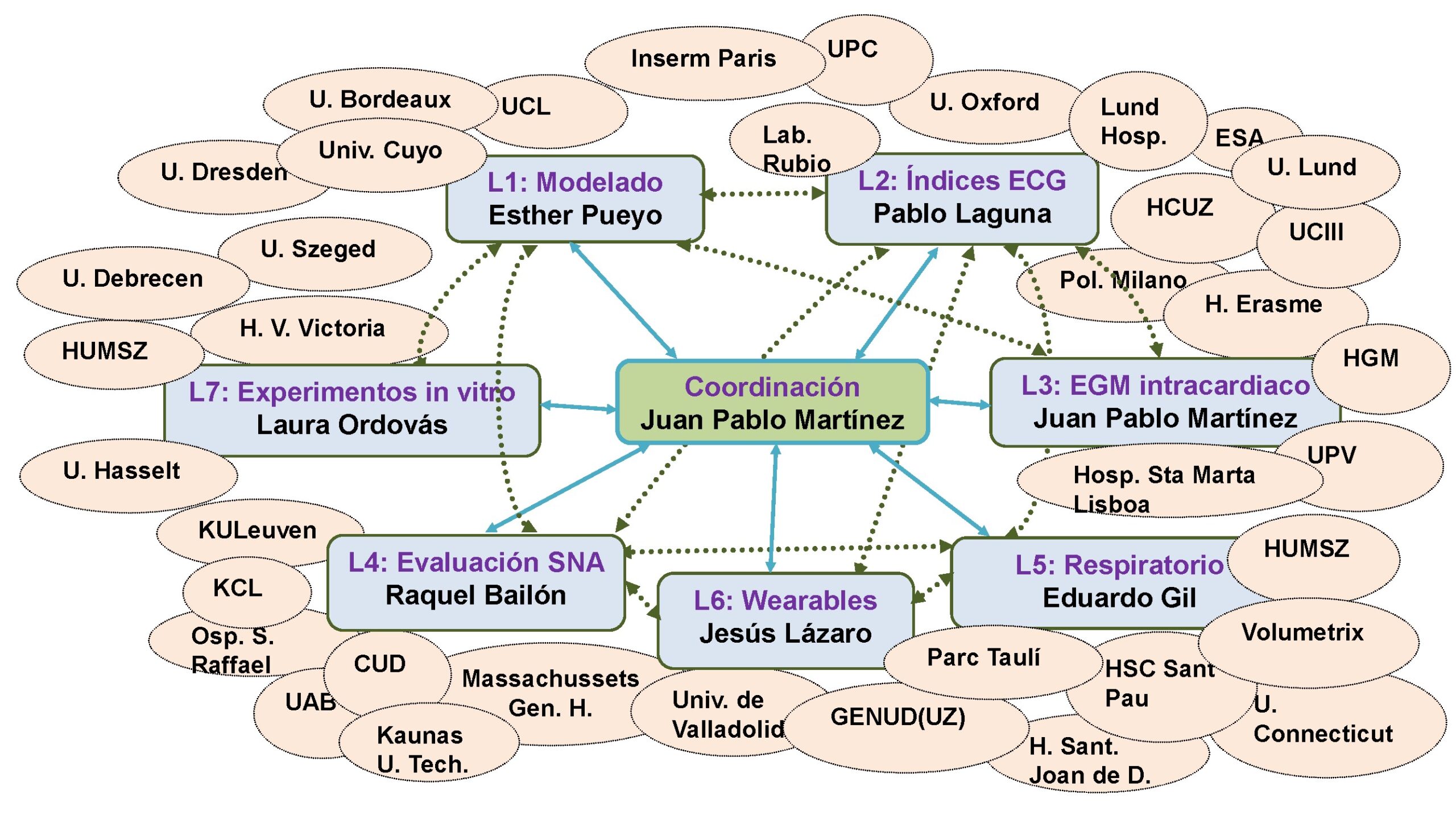
All this is embodied in 11 application domains that constitute the lines of work of the group:
The lines of research addressed by the Group pursued basic scientific and technological progress through the development of algorithms and methods to improve diagnosis, prognosis and therapy application in cardiorespiratory and autonomic nervous system diseases, as well as a better understanding of physiology pathological or not pathological situations (drowsiness, stress, microgravity, etc).